Highlight
- Speed & Efficiency: AI reduces drug discovery timelines by 25% and clinical trial costs by up to 70%
- Predictive Power: Machine learning models predict drug-target interactions and patient responses with 85%+ accuracy
- Cost Savings: Pharmaceutical AI implementation saves companies $26 billion annually in development costs
- Market Growth: The AI in pharma market is projected to reach $350-410 billion by 2025, with 75% of companies prioritizing generative AI
- Real ROI: Early adopters report 20% improvements in marketing effectiveness and 30-50% reductions in equipment downtime
- Future Ready: Regulatory frameworks are evolving to accommodate AI, with FDA launching AI discussion groups and approval pathways
Introduction
Last month, I watched a pharmaceutical executive’s jaw drop when I showed him the numbers: his company’s AI-powered drug discovery program had just identified three promising compounds in two weeks—work that previously took his team six months.
The pharmaceutical industry is at a tipping point. While traditional drug development burns through $2.6 billion and 15 years per approved medication, AI-enabled companies are rewriting the rules entirely. McKinsey[1] estimates that generative AI alone could save pharma $60-110 billion annually.
Here’s the reality: 75% of pharmaceutical companies have already made AI a strategic priority for 2025. Yet 42% of current AI initiatives fail to meet ROI expectations. The difference between success and failure isn’t the technology—it’s the implementation strategy.
From my experience helping pharmaceutical companies implement data analytics solutions, the winners share common traits: they start with clear business objectives, invest in data quality first, and build regulatory compliance into every AI in pharmaceutical industry project from day one.
AI in Pharma – An Overview of Opportunities and Impact
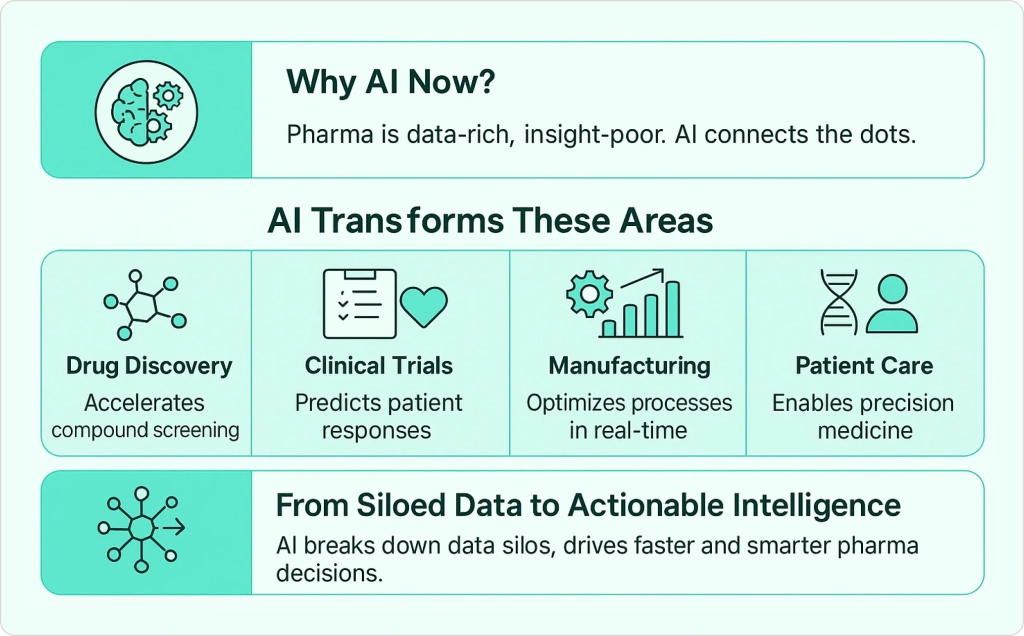
What Makes AI Essential for Pharma Now?
Artificial intelligence in the pharmaceutical context means using advanced algorithms and machine learning models to perform tasks like analyzing chemical data, predicting clinical outcomes, or automating workflows that traditionally relied on human expertise alone.
The pharmaceutical industry has historically been data-rich but insight-poor. Labs generate massive amounts of research data, clinical trials produce complex patient information, and manufacturing systems create operational metrics—yet much of this valuable information sits in silos, underutilized.
AI in pharmaceutical industry transforms this scattered data into actionable intelligence, enabling companies to make faster, more informed decisions across drug development, clinical trials, manufacturing, and patient care.
AI turns this scattered data into actionable intelligence.
Key Benefits Driving Pharmaceutical AI Adoption
Faster Drug Discovery and Development: AI drastically cuts down R&D timelines through virtual screening that can test millions of compounds in silico, narrowing down candidates in weeks rather than years. According to recent studies, AI can reduce drug discovery times by approximately 25%, helping companies bring treatments to market faster.
Substantial Cost Savings: The efficiency gains translate directly to cost reduction. Industry studies project AI could save pharmaceutical companies $25 billion in clinical development alone[2] by automating processes and reducing late-stage trial failures. PwC projects that AI could boost pharma profits by $254 billion by 2030.
Improved Success Rates: AI’s predictive power helps identify promising drug targets with higher success probability and flags potential failures early, reducing expensive late-stage trial setbacks. AI-optimized clinical trial design can lead to 80% shorter trial timelines in some cases.
Personalized Medicine: AI enables tailoring treatments to patient sub-groups through genomics and big data analysis, improving both efficacy and safety. This leads to better patient outcomes—the ultimate goal of pharmaceutical innovation.
Why the Urgency Now?
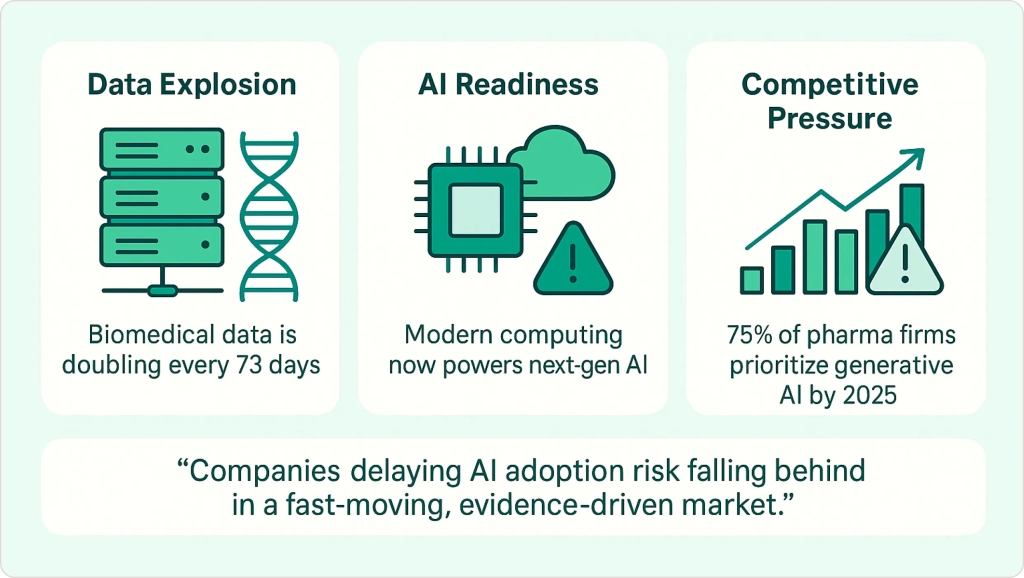
Several factors are accelerating AI adoption in pharma right now. The explosion of biomedical data makes manual analysis impossible—genomic data, electronic health records, and research papers are growing exponentially. Advances in computing power now enable complex AI models that weren’t feasible just five years ago.
The competitive pressure is real. By 2025, 75% of pharmaceutical companies have made generative AI a strategic priority[3]. Companies that lag in AI adoption risk being left behind as success stories emerge and prove the technology’s value.
Top Applications and Use Cases of AI in the Pharmaceutical Industry
From my experience helping pharmaceutical companies implement AI-driven analytics solutions, AI in pharmaceutical industry is making its mark at every stage of the pharmaceutical lifecycle. Here are the most impactful use cases I’ve observed:
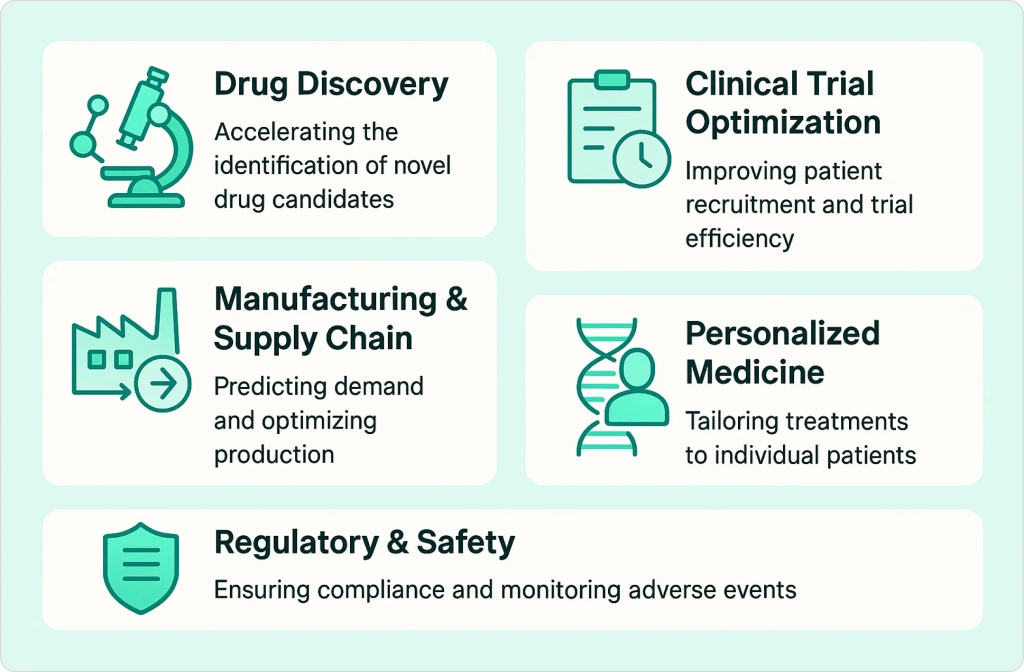
Drug Discovery & Preclinical Research
Virtual Compound Screening: AI algorithms analyze vast chemical and biological datasets to identify new drug candidates faster than traditional methods. Deep learning models like generative adversarial networks (GANs) can actually “create” novel molecules by predicting chemical structures likely to be effective.
Target Identification: Machine learning helps predict which molecules are likely to work and which aren’t by analyzing ADMET properties—absorption, distribution, metabolism, excretion, and toxicity—using AI to avoid dead-ends early in the process.
Success Story: Insilico Medicine used AI to design a novel anti-fibrosis drug and brought it to Phase I trials in under 18 months, roughly 50% of the time normally needed. This demonstrates real impact beyond theoretical possibilities.
AI is essentially becoming the “creative scientist,” proposing novel compounds that human chemists might not consider, significantly expanding the possibility space for drug discovery.
Clinical Trial Optimization
Patient Recruitment & Matching: AI helps identify optimal trial participants using electronic health record data and even social media or genetic information, improving recruitment speed and diversity. About 30% of trial delays stem from recruitment issues—AI can substantially mitigate this bottleneck.
Real-Time Trial Monitoring: During trials, AI monitors incoming data for safety signals or efficacy trends, potentially identifying if a trial is likely to succeed or fail early, allowing sponsors to adjust protocols or halt studies to save money.
Predictive Analytics: Industry studies show AI-enabled trial processes can save up to 70% of trial costs and shorten timelines by 50-80%. McKinsey reported that AI-driven trial operations led to 80% timeline reductions in some cases[6].
The impact extends beyond cost savings. Better patient matching through AI leads to more diverse, representative study populations, ultimately producing better drugs for broader patient groups.
Manufacturing & Supply Chain
Predictive Maintenance: AI predicts equipment failures from sensor data, preventing downtime that could disrupt production schedules. In pharmaceutical manufacturing where compliance and quality are critical, unexpected equipment failures can be extremely costly.
Quality Control: Computer vision systems detect defects on production lines that human inspectors might miss, ensuring consistent product quality while reducing manual inspection time.
Supply Chain Optimization: AI forecasts demand and optimizes inventory management—crucial for avoiding drug shortages. During COVID-19, companies with AI-driven supply chain management showed greater resilience against disruptions.
Results: One case study found that AI-driven scheduling improved throughput by 20% and cut changeover times by 22% for a major pharmaceutical manufacturer. McKinsey research[5] shows that top AI-enabled pharma factories have 14 times lower quality costs than peers.
Results: One case study found that AI-driven scheduling improved throughput by 20% and cut changeover times by 22% for a major pharmaceutical manufacturer. Recent analysis shows that AI-enabled pharma factories can achieve up to 10% cost reductions while significantly improving throughput[5].
Personalized Medicine & Patient Engagement
Precision Treatment Selection: AI analyzes patient data—genomics, proteomics, health records—to segment patients and predict who will respond to what treatment. For cancer care, AI can identify biomarkers that signal a patient’s likely response, helping pair patients with the most effective clinical trial treatments.
Digital Therapeutics: AI enables patient monitoring through wearables and mobile apps, tracking adherence and detecting adverse events early. Healthcare data analytics powered by AI helps pharmaceutical companies monitor patients in real-world settings and optimize treatment outcomes.
Real-World Evidence: AI scans social media and databases for adverse event signals, enabling faster safety monitoring than traditional voluntary reporting systems. The FDA’s Sentinel Initiative uses AI to analyze healthcare data for drug safety issues in near real-time.
This bridges to an important but less flashy use case: ensuring patient safety through proactive monitoring rather than reactive responses.
Regulatory Compliance & Pharmacovigilance
Automated Documentation: Natural language processing helps auto-generate portions of regulatory submissions, checking for errors and ensuring consistency across complex approval documents. This accelerates the traditionally labor-intensive approval process.
Safety Signal Detection: AI systems monitor adverse event reports and real-world data to detect safety signals sooner than manual processes, helping pharma companies respond proactively and maintain regulatory compliance.
The FDA’s Sentinel Initiative exemplifies how AI enhances safety monitoring—analyzing healthcare data for drug safety issues that previously relied on slower voluntary reporting systems.
AI in Pharmaceutical Marketing and Sales – A New Frontier
Beyond R&D and production, AI in pharmaceutical industry is increasingly transforming how pharmaceutical companies market their products and engage with healthcare providers and patients. This represents a significant opportunity that many companies are just beginning to explore.
Intelligent HCP Targeting
Data-Driven Sales Strategy: Machine learning analyzes prescribing patterns, patient demographics, and healthcare provider preferences to segment HCPs and tailor outreach approaches. Sales representatives get data-driven suggestions on whom to prioritize and what to emphasize during visits.
From conversations with pharma sales teams, I’ve learned that traditional sales approaches often waste significant time on unproductive calls. AI-powered HCP engagement can deliver personalized content in “30 minutes instead of 3 days” for follow-ups, according to industry case studies. This means sales reps can spend more time actually selling rather than preparing materials.
Personalized Content & Outreach
Generative AI for Compliance: Generative AI models can be fine-tuned on approved pharmaceutical content to produce on-brand, compliant marketing text quickly. This saves marketers substantial time in content creation and localization across different markets.
Multilingual Content Creation: AI can auto-translate and adapt marketing materials while ensuring medical accuracy and regulatory compliance—a process that previously took weeks when done manually.
Omnichannel Marketing Optimization
Channel & Timing Intelligence: Predictive analytics determine the best channel and timing to reach specific healthcare providers or patient groups. AI might identify that certain clinics respond better to webinars versus in-person visits, or that specific patient groups engage more through mobile apps than traditional materials.
AI Chatbots and Virtual Assistants
24/7 Support: Many pharmaceutical companies deploy AI chatbots to handle common inquiries from both healthcare providers and patients. For example, a bot on a pharma website can answer “What’s the dosage for Drug X for pediatric patients?” using the company’s approved database.
Clinical Trial Matching: AI-powered systems help match patients to appropriate clinical trials, streamlining the recruitment process while providing valuable service to patients seeking treatment options.
Medical-Legal-Regulatory (MLR) Acceleration
Compliance Automation: AI tools pre-screen promotional content for compliance issues, checking claims against approved references and verifying that only validated language is used. This reduces the traditional back-and-forth with legal teams, potentially cutting content approval time from 8 weeks to days.
Results: Early pilots show approximately 20% improvement in marketing ROI when using advanced AI analytics for campaign optimization and audience targeting.
This marketing transformation represents a competitive advantage for companies that implement it effectively, enabling more personalized and efficient campaigns while maintaining strict compliance standards.
Challenges of Implementing AI in Pharma (and How to Overcome Them)
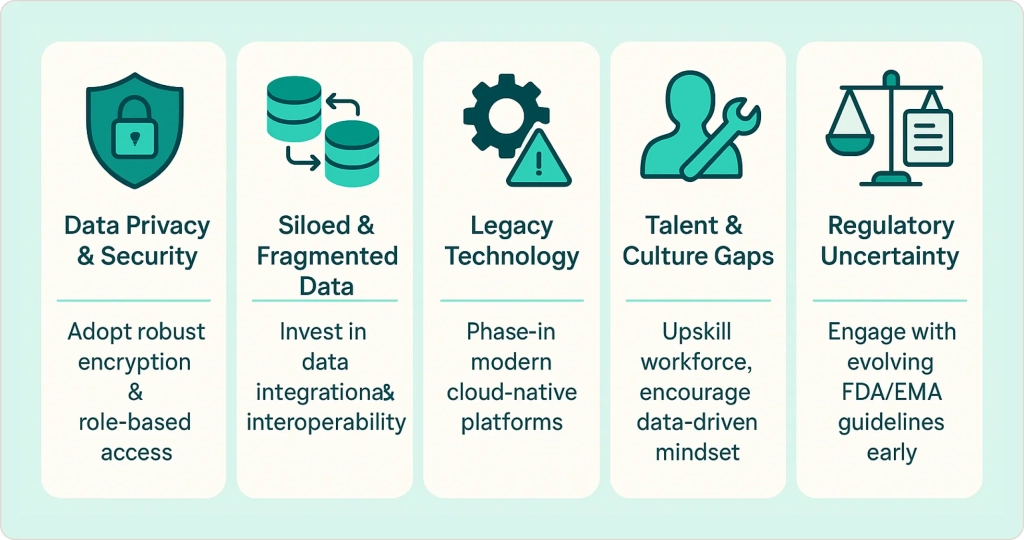
While the potential of AI in pharmaceutical industry is immense, deploying these technologies successfully requires addressing several significant challenges. From my experience working with pharmaceutical companies on data analytics implementation, understanding these hurdles is the first step to overcoming them.
Data Privacy & Security
The Challenge: Pharmaceutical companies handle sensitive patient data and proprietary research information. AI systems require large datasets, raising HIPAA compliance and data breach concerns.
The Solution: Implement robust data governance from day one. Use compliant data collection methods, anonymize datasets for AI training, and partner with HIPAA-compliant cloud providers. Consider federated learning techniques where AI models learn without data leaving secure environments.
Siloed and Fragmented Data
The Challenge: Pharma data often exists in separate systems that don’t communicate. Poor data quality leads to “garbage in, garbage out” issues, with data scientists spending 80% of their time on cleaning rather than analysis.
The Solution: Invest in data integration—adopt modern data warehouses that consolidate information. Implement FAIR data principles (Findable, Accessible, Interoperable, Reusable) and start AI projects with thorough data audits.
Legacy Technology & Integration Complexity
The Challenge: Many pharmaceutical IT systems weren’t built for AI integration. Deploying AI might require costly infrastructure upgrades or risk disrupting ongoing operations.
The Solution: Take a phased modernization approach. Move gradually to cloud platforms that can host AI capabilities and use middleware to connect AI tools with existing systems. Plan and budget for necessary IT upgrades.
Talent & Culture Gaps
The Challenge: AI requires new skill sets that are in short supply. Internal resistance may come from scientists skeptical of “black-box” algorithms or concerned about job displacement.
The Solution: Combine upskilling with strategic hiring. Build cross-functional teams including both AI experts and domain specialists. Address the “fear factor” by emphasizing that AI augments human expertise and sharing internal success stories.
Regulatory Uncertainty & Compliance
The Challenge: Regulatory bodies are still developing AI oversight frameworks. Companies worry about how regulators will view AI-determined outcomes, especially for critical decisions.
The Solution: Engage with regulators early in pilot projects. Maintain detailed documentation of AI processes for auditability and keep humans in the loop for final decisions. Stay current with evolving guidelines as the FDA develops clearer approval pathways.
How to Successfully Implement AI in a Pharmaceutical Organization
Based on our experience helping pharmaceutical companies implement AI solutions, successful AI in pharmaceutical industry implementation requires equal parts strategy, technology, and change management. Here’s a practical roadmap:
Step 1: Define Clear Goals and Use Cases
Don’t adopt AI for the sake of appearing innovative. Identify specific pain points or opportunities with measurable outcomes—such as “reduce trial recruitment time by 50%” or “improve manufacturing efficiency by 15%.” This alignment ensures stakeholder buy-in and provides clear success metrics from the start.
One of the biggest failure factors is lack of focus. Companies that succeed with AI tie each project directly to business objectives like reducing costs, accelerating timelines, or improving compliance.
Step 2: Secure Leadership Buy-In and Build Cross-Functional Teams
Successful AI projects need executive champions who can allocate budget and drive cultural acceptance. Create an AI task force that includes IT/data experts, pharmacologists, process owners, and compliance officers. This ensures solutions fit real-world workflows and meet regulatory requirements from day one.
AstraZeneca’s success came from enterprise-wide AI strategy and cross-functional integration rather than isolated pilot projects. When everyone—from data scientists to domain experts—collaborates, AI solutions become both technically sound and practically usable.
Step 3: Audit and Prepare Your Data
Since AI outcomes depend entirely on data quality, start with a comprehensive data inventory. Identify your data sources (research data, clinical trials, sales data), address quality issues, and integrate them where possible. This might involve setting up centralized data platforms or implementing data engineering best practices.
Remember: if 40% of pharma AI projects stall due to data issues, investing time in data preparation is non-negotiable. Start with smaller, high-quality datasets if necessary, then expand systematically.
Step 4: Start with Pilot Projects and Iterate
Choose projects with reasonable scope and clear ROI potential. For example, apply AI to one clinical trial for recruitment optimization, or implement predictive maintenance on one production line. Set specific success criteria and implement in controlled settings.
Use an agile approach—incorporate feedback from end-users and improve the model iteratively. This builds internal credibility and helps get skeptics on board when they see tangible results. Plan for scaling successful pilots from the beginning to avoid “pilot purgatory.”
Step 5: Ensure Regulatory and Ethical Compliance
Consult your regulatory affairs team early in any AI project. If AI touches GxP processes, implement proper validation protocols. Establish ethical AI frameworks that ensure diverse data sources, include human oversight for critical decisions, and maintain transparency through decision audit trails.
By building compliance into the process rather than treating it as an afterthought, you minimize risk and make regulators more comfortable with your AI applications.
Step 6: Invest in Skills and Change Management
Beyond technology, successful AI implementation requires people to trust and effectively use new tools. Provide training on data literacy and basic AI concepts for existing employees. Address cultural resistance by communicating that AI augments rather than replaces human expertise.
Step 7: Scale Up and Integrate into Business Processes
After successful pilots, create standardized protocols for new AI projects. Integrate AI systems with existing workflows and monitor performance continuously. Treat AI implementation as an ongoing capability rather than a one-time project, updating models and incorporating new data as the organization evolves.
Future Trends – What’s Next for AI in Pharma?
Looking ahead, several emerging trends will shape how AI in pharmaceutical industry transforms pharmaceutical development and operations:
AI + Robotics & Lab Automation
The convergence of AI with robotics is creating “Labs of the Future” where AI algorithms not only design experiments but robots physically conduct them. This enables “closed-loop drug discovery” where AI generates hypotheses, tests them automatically, and continuously refines results with minimal human intervention.
Generative AI for Protein Design
Beyond small-molecule discovery, generative AI is designing complex biologics like proteins and antibodies. DeepMind’s AlphaFold breakthrough in protein folding has enabled new approaches to structure-based drug design, with generative models potentially creating AI-designed cancer therapeutics and personalized vaccines.
Digital Twins for Clinical Development
AI will create digital simulations of patients or clinical trials, allowing researchers to test designs virtually before implementation. Real-world data integration through AI will complement traditional trials, providing comprehensive pictures of drug impact across diverse populations.
Regulatory Evolution and AI Democratization
By 2025-2026, expect clearer regulatory frameworks from the FDA and EMA for AI in pharmaceutical development. Simultaneously, AI tools will become accessible to frontline employees through user-friendly interfaces, with lab notebooks and clinical systems featuring built-in AI suggestions.
Conclusion: The AI-Powered Future of Pharmaceutical Innovation
The evidence is overwhelming: AI in the pharmaceutical industry has moved from experimental technology to business necessity. Companies implementing AI across their value chain are achieving remarkable results—25% faster drug discovery, 70% cost reductions in clinical trials, and 20% improvements in marketing effectiveness.
But success isn’t guaranteed. The 42% of AI in pharmaceutical industry initiatives that fail to meet ROI targets share common characteristics: rushed implementation without proper data preparation, lack of cross-functional collaboration, and insufficient attention to regulatory compliance.
The companies winning with AI follow a different playbook. They start with clear business objectives, invest heavily in data quality and team training, and build compliance into their processes from day one. Most importantly, they view AI as a fundamental capability rather than a side project.
The future belongs to pharmaceutical companies that embrace AI as a core competency. By 2025, the gap between AI-enabled and traditional pharmaceutical companies will become insurmountable. Early movers will dominate not just through better drugs, but through more efficient operations, smarter marketing, and deeper patient insights.














