TL;DR:
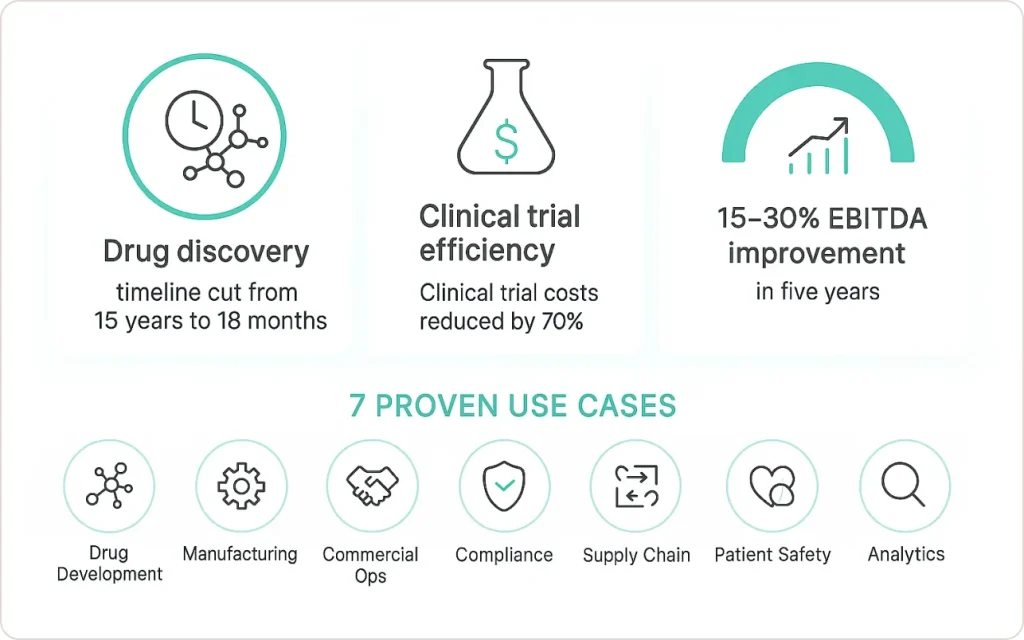
- Drug discovery timelines cut from 15 years to 18 months using AI-powered analytics platforms
- Clinical trial costs reduced by 70% through predictive patient selection
- Manufacturing efficiency gains of 30-50% via real-time quality control
- 15-30% EBITDA improvement within 5 years for pharma companies adopting advanced analytics
The $2.6 Billion Problem
Developing a single FDA-approved drug costs pharmaceutical companies an average of $2.6 billion and takes 15 years from discovery to market. For every successful medication, ninety percent of candidates fail during clinical trials, often after hundreds of millions in investment.
This brutal economic reality is changing. Data analytics in pharmaceutical industry operations has emerged as the most significant competitive advantage since the genomics revolution. Companies leveraging advanced analytics are cutting discovery timelines by 25%, reducing clinical trial costs by up to 70%, and improving patient outcomes through precision medicine.
McKinsey projects that advanced analytics can boost pharmaceutical EBITDA by 15-30% within five years, with potential acceleration to 45-75% over a decade. These aren’t incremental improvements; they represent fundamental transformation in how drugs are discovered, developed, manufactured, and delivered.
This guide examines seven critical use cases where pharma analytics delivers measurable ROI, backed by real-world implementations from industry leaders.
Quick Answer
Pharmaceutical companies using advanced analytics cut drug discovery timelines from 15 years to 18 months, reduce clinical trial costs by 70%, and achieve 15-30% EBITDA improvements within five years. This guide examines seven proven use cases where data analytics delivers measurable ROI across drug development, manufacturing, commercial operations, patient safety, compliance, and supply chain management.
What Is Data Analytics in Pharmaceutical Industry?
Data analytics in pharmaceutical industry applications encompass the systematic collection, integration, and analysis of vast datasets generated throughout the drug lifecycle, from molecular research through post-market surveillance.
Modern pharmaceutical operations generate unprecedented data volumes. A single Phase III clinical trial produces terabytes of patient data. Manufacturing facilities generate millions of quality control measurements daily. Electronic health records capture real-world treatment outcomes for millions of patients.
Pharmaceutical data solutions transform this raw information into actionable intelligence using machine learning, natural language processing, and predictive modeling. These technologies identify patterns invisible to traditional analysis, enabling everything from virtual drug candidate screening to predicting supply chain disruptions before they occur.
Research from McKinsey shows that companies using advanced analytics are 23 times more likely to outperform competitors in customer acquisition and retention. Organizations implementing comprehensive data analytics strategies gain competitive advantages that compound over time through faster decision-making and improved operational efficiency.
1. Accelerating Drug Discovery Through Predictive Analytics
Traditional drug discovery required researchers to synthesize and test thousands of chemical compounds, with 90% failing before reaching human trials. This trial-and-error approach consumed years and hundreds of millions before identifying viable candidates.
Pharmaceutical data analytics now enables virtual compound screening where machine learning algorithms analyze massive chemical and biological datasets to identify promising candidates before physical synthesis.
Insilico Medicine progressed from novel target discovery to preclinical candidate in just 18 months, spending only $2.6 million, a fraction of traditional timelines and budgets. Their AI platform analyzed millions of potential molecular structures to identify optimal drug designs for pulmonary fibrosis treatment.
Predictive modeling for drug-target interactions has reached 85%+ accuracy in predicting how molecules will bind to disease targets. Pfizer uses these pharmaceutical data solutions to prioritize compounds most likely to succeed in clinical testing, dramatically reducing failed experiments.
Natural language processing systems analyze millions of published research papers and patents to uncover hidden connections between diseases, genes, and potential treatments. This pharma data analytics approach, similar to how AI transforms healthcare operations, has identified numerous drug repurposing candidates that successfully entered clinical trials.
Key outcomes:
- Virtual screening of billions of compounds in weeks versus years
- 40-60% reduction in preclinical development costs
- Early identification of safety issues before expensive synthesis
- Discovery of repurposing opportunities for existing medications
Organizations looking to implement similar capabilities benefit from data science consulting services that provide the technical expertise and infrastructure needed for advanced predictive modeling.
2. Optimizing Clinical Trials With Data-Driven Patient Selection
Clinical trials consume 40-50% of total drug development costs, with patient recruitment representing the single biggest bottleneck. Traditional approaches relied on manual chart review, slow, expensive, and often missing ideal candidates.
Commercial analytics in pharma clinical operations transform trial design through predictive algorithms that analyze electronic health records and genetic databases to identify patient populations most likely to respond to treatment. This precision targeting increases success rates while reducing required trial sizes.
Novartis processes over 9 terabytes of data from 80+ sources through their unified analytics platform. This pharmaceutical data management infrastructure reduced the time to set up new analytics use cases from two weeks to one day, enabling rapid adaptation during active trials.
Real-time monitoring enables continuous analysis of incoming results. If data reveals a subgroup responding exceptionally well, adaptive trial designs pivot to focus resources where they’ll have maximum impact.
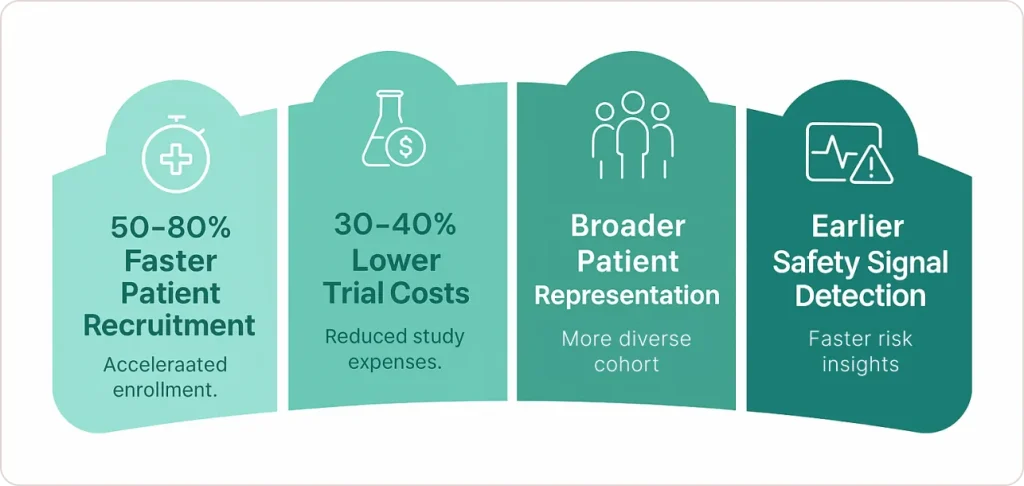
Measurable results:
- 50-80% reduction in patient recruitment timelines
- 30-40% decrease in overall trial costs
- More diverse patient populations improving drug effectiveness
- Earlier detection of safety signals preventing late-stage failures
The predictive capabilities mirror those used in healthcare analytics for improving patient outcomes, where similar machine learning approaches identify at-risk populations and optimize intervention timing.
3. Enhancing Manufacturing Efficiency and Quality Control
Pharmaceutical manufacturing operates under stringent quality requirements where even minor deviations can necessitate batch disposal, potentially destroying millions in product. Traditional quality control relied on periodic testing, often discovering problems only after significant production.
Data analytics in pharmaceutical industry manufacturing enables predictive quality control that detects issues before they compromise entire batches. Sensor data from temperature controls, mixing equipment, and packaging lines feeds into machine learning models that predict deviations before they occur.
An AI-powered platform for continuous manufacturing that detects abnormal data points in real-time. The system leverages machine learning to identify anomalies while pinpointing root causes, preventing costly production disruptions.
Predictive maintenance analyzes sensor data to predict equipment failures days or weeks in advance. This approach reduces downtime by 30-50% while extending equipment life through optimized maintenance timing.
Pharmaceutical data management systems track materials from suppliers through production to distribution, enabling:
- Real-time inventory optimization reducing carrying costs by 15-20%
- Demand forecasting that prevents shortages and overproduction
- Quality traceability ensuring rapid response to safety issues
- Supplier performance analytics identifying reliability risks
GSK’s analytics platform monitors global manufacturing operations, analyzing millions of data points daily to maintain consistent quality while identifying efficiency opportunities. This data driven pharma approach helped them achieve 95%+ on-time-in-full delivery rates.
4. Revolutionizing Sales and Marketing With Commercial Analytics
Pharmaceutical marketing differs fundamentally from consumer goods. Success requires influencing physician prescribing behavior within strict regulatory constraints. The challenge: personalizing outreach to 800,000+ US physicians while maintaining compliance.
Commercial analytics in pharma solves this through sophisticated segmentation and next-best-action recommendations. Analytics platforms combine internal sales data with external market intelligence, using machine learning to recommend optimal physician engagement strategies.
Novartis exemplifies data driven pharma in commercial operations. Sales representatives receive personalized talking points based on each doctor’s patient population and prescribing history, derived from anonymized claims data and treatment pattern analysis.
Pharmaceutical data solutions track marketing attribution across touchpoints: digital advertising, medical conferences, journal publications, and patient education programs, revealing which investments drive actual prescription growth.
Measurable outcomes:
- 25-30% improvement in sales force effectiveness through territory optimization
- 40-50% better marketing ROI by eliminating underperforming channels
- 15-20% increase in physician engagement through personalized content
- Faster market access through competitive intelligence
Patient adherence analytics identify when individuals are most likely to discontinue treatment. Advanced programs using pharmaceutical data analytics have increased medication persistence by 35% through timely outreach to at-risk patients.
5. Ensuring Patient Safety Through Advanced Pharmacovigilance
Traditional pharmacovigilance relied on spontaneous adverse event reports submitted by healthcare providers. This reactive approach often missed rare side effects, sometimes taking years to identify critical safety signals.
Modern pharmaceutical data analytics integrates diverse information streams to identify safety signals earlier. Johnson & Johnson’s platform analyzes adverse event databases, electronic health records, insurance claims, and social media mentions to detect potential safety concerns invisible in any single data source.
Natural language processing analyzes unstructured clinical notes, patient forums, and social media conversations. Pharmaceutical data solutions identify adverse event discussions that never reach formal reporting channels, often detecting usability issues months before they appear in regulatory databases.
Predictive safety analytics analyze patient characteristics, medication interactions, and genetic factors to predict who faces elevated risk. This enables proactive monitoring and risk mitigation for vulnerable populations.
The FDA’s Sentinel Initiative analyzes electronic health data from 178 million patients across distributed networks, enabling near-real-time safety monitoring. When concerns emerge, the system rapidly assesses whether observed patterns represent genuine safety signals.
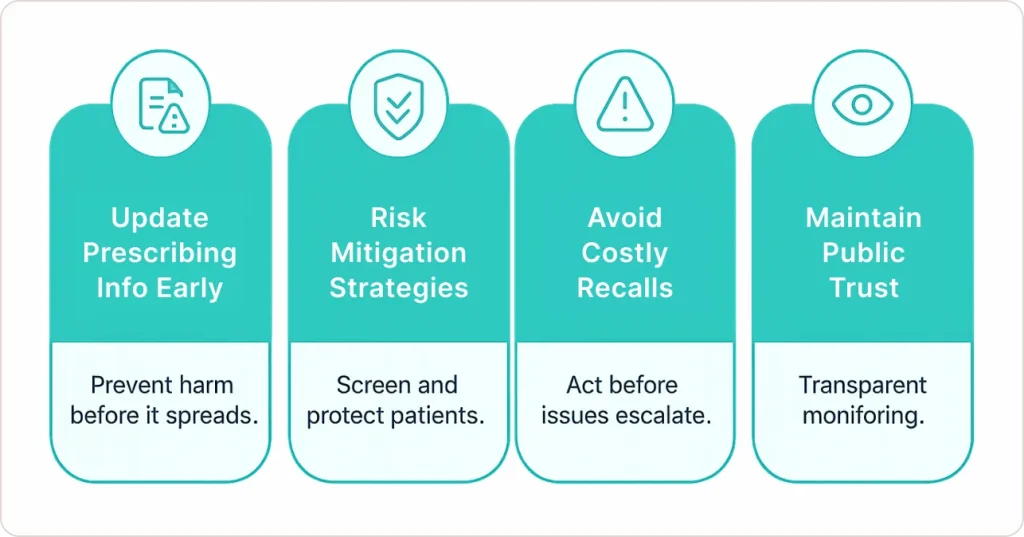
Critical benefits:
- Updating prescribing information before widespread harm occurs
- Implementing risk mitigation strategies like patient screening
- Avoiding recalls through proactive management
- Maintaining public trust through transparent monitoring
Analytics platforms have detected cardiovascular and other safety signals months before traditional methods, enabling label updates that prevented thousands of adverse events. The predictive approaches used mirror those in AI-powered healthcare analytics that identify patient risks hours before clinical deterioration becomes apparent.
6. Strengthening Regulatory Compliance and Risk Management
Pharmaceutical companies operate in one of the most heavily regulated industries globally. Data analytics in pharmaceutical industry operations enables proactive compliance management, transforming reactive audit responses into continuous risk monitoring.
Sanofi demonstrates innovative pharma data analytics in regulatory submissions. They use natural language generation to automatically convert clinical trial data into regulatory documents, a process that once required weeks now completes in minutes.
Continuous monitoring of manufacturing parameters against validated specifications flags deviations immediately. Quality teams address problems within hours, preventing batch losses and regulatory violations.
Pharmaceutical data analytics platforms include automated audit trails, electronic signature validation, and anomaly detection that identifies suspicious data patterns. These systems detect unauthorized modifications or unusual access patterns, critical for avoiding regulatory findings during inspections.
Privacy compliance represents an increasingly complex challenge. HIPAA in the US, GDPR in Europe, and similar regulations worldwide require strict data protection. Pharmaceutical data solutions include automated data anonymization, access controls, and breach detection ensuring compliance while enabling analytics.
Organizations implementing comprehensive compliance analytics report:
- 25-40% reduction in compliance costs
- Significantly improved audit readiness and inspection outcomes
- Early identification and resolution of issues before they escalate
- Streamlined regulatory reviews through demonstrated compliance systems
7. Optimizing Supply Chain Resilience and Distribution
Pharmaceutical supply chains present unique complexities: temperature-sensitive products, strict serialization requirements, and the critical need to prevent shortages that could endanger patient health.
Data analytics in pharmaceutical industry supply chains begins with demand forecasting. By analyzing prescription trends, disease prevalence patterns, demographic shifts, and weather data, pharmaceutical data solutions predict demand with remarkable accuracy.
Merck achieved a 95% on-time-in-full delivery rate while reducing inventory carrying costs by 15% through commercial analytics in pharma supply operations. The key: predictive analytics that balanced production efficiency against service requirements.
Temperature-sensitive biologics require strict climate control throughout distribution. IoT sensors generate continuous temperature data, with analytics platforms immediately flagging deviations that could compromise product quality.
Supply risk analytics identify vulnerabilities before they disrupt operations. By analyzing supplier performance, geopolitical factors, and natural disaster risks, pharmaceutical data solutions enable contingency planning.
COVID-19 vaccine distribution provided a stress test for pharma analytics. Johnson & Johnson used their supply chain analytics platform to track virus hotspots in real-time, dynamically allocating vaccine supplies where need was greatest.
Pharmaceutical companies implementing comprehensive supply chain analytics typically achieve 10-20% cost reductions while simultaneously improving service levels.
The Future of Pharmaceutical Analytics
The data analytics in pharmaceutical industry landscape continues evolving rapidly. Emerging trends include real-time data streaming enabling instantaneous decision-making, federated learning allowing collaborative analytics while preserving privacy, and digital twins creating virtual representations of manufacturing processes.
The organizations leading pharmaceutical innovation in 2030 will be those that master pharma analytics today. McKinsey’s projection of 15-30% EBITDA gains represents hundreds of millions in value for major pharmaceutical companies.
Beyond financial returns, pharmaceutical data solutions enable breakthroughs that improve and extend human lives. Clinical trials that once took years now complete in months. Drugs that might have failed succeed because analytics identified the right patient populations. Supply chains that struggled with shortages now predict and prevent disruptions.
Taking Action: Your Next Steps
Data analytics in pharmaceutical industry operations has evolved from competitive advantage to business imperative. Companies embedding pharma analytics into every function are capturing disproportionate value.
The seven use cases explored here represent the foundation of modern pharmaceutical operations. Organizations mastering these applications will lead the industry’s next decade of innovation.
Three priorities for getting started:
- Build robust data infrastructure that integrates diverse sources and ensures quality
- Develop analytics talent understanding both data science and pharmaceutical operations
- Start with high-impact use cases that demonstrate measurable ROI and build momentum
The transformation powered by pharma data analytics isn’t just about technology. It’s about fundamentally reimagining how pharmaceutical companies discover, develop, manufacture, and deliver life-saving medications.














